Description
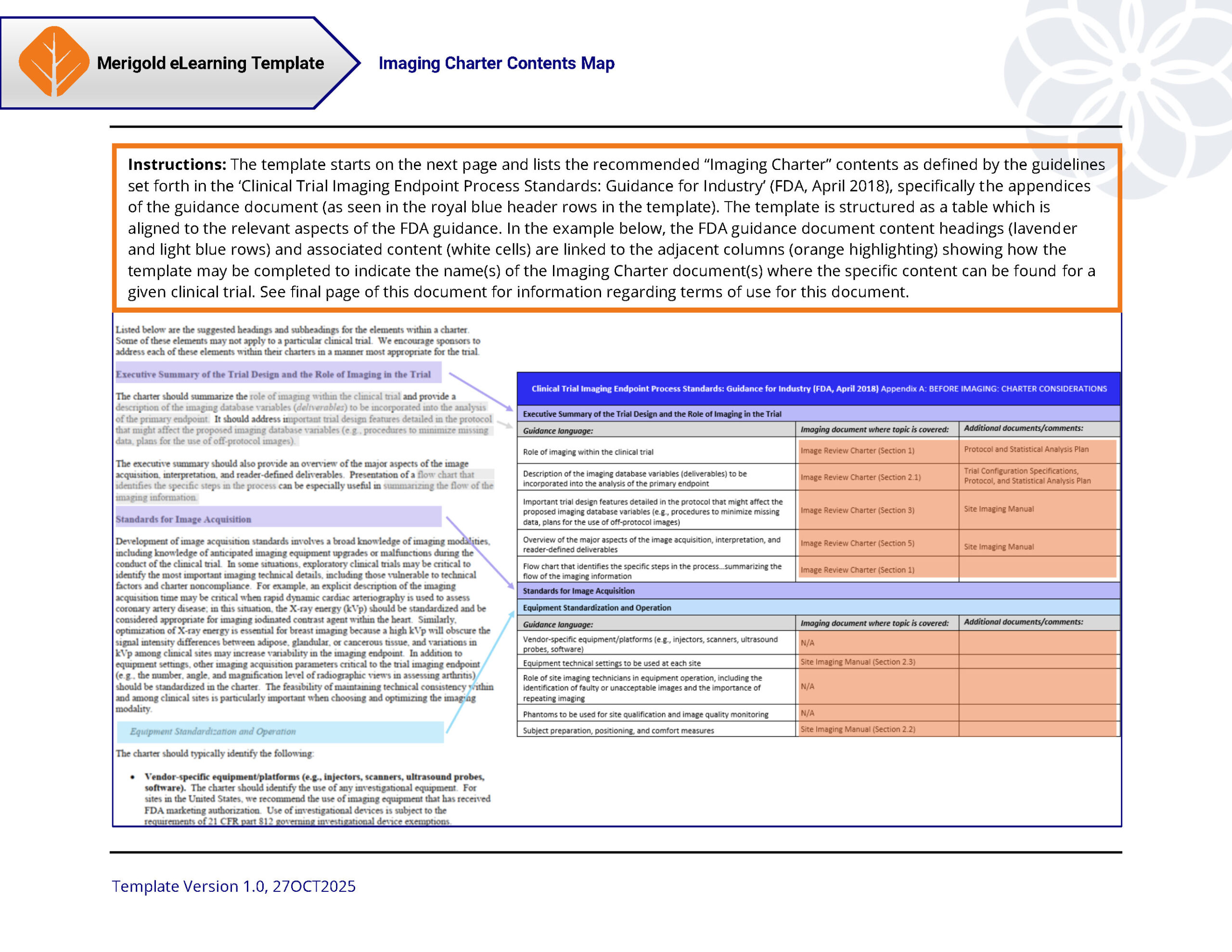
This fillable PDF document template lists the recommended “Imaging Charter” contents as defined by the guidelines set forth in the ‘Clinical Trial Imaging Endpoint Process Standards: Guidance for Industry’ (FDA, April 2018), specifically the appendices of the guidance document. The template is structured as a table which is aligned to the relevant aspects of the FDA guidance. This template is appropriate for anyone responsible for the planning and/or conduct of a clinical trial which utilizes an Imaging Charter where medical imaging is used for disease staging and/or assessment (e.g., trial sponsors, clinical research organizations/providers). The download link is valid for 72 hours from purchase and limited to three (3) downloads. Attempting to bypass these restrictions is prohibited.